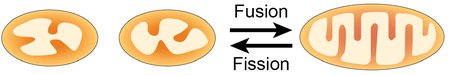
CELLULAR FUNCTIONS OF MITOCHONDRIAL DYNAMICS
Eukaryotic cells, such as hepatocytes, can have hundreds of mitochondria. However, each mitochondrion is not an isolated, autonomous organelle. Mitochondria within the population are dynamic and continually fuse and divide, leading to exchange of membranes and internal contents. Why do cells expend energy to maintain their mitochondria in such a dynamic state? One clearly established reason is that the equilibrium between fusion and fission rates regulates mitochondrial morphology, which typically manifests a mixture of tubules and spheres. Cells with unopposed fusion have overly long and interconnected mitochondria; conversely, cells with unopposed fission have fragmented mitochondria. Morphology can indeed affect mitochondrial function, but dynamics also appears to regulate mitochondrial physiology through other mechanisms.
We have found that it is the balance between fusion and fission, rather than their absolute rates, that is important for mitochondrial physiology. Defects in mitochondrial physiology arise when these rates are unbalanced. Fusion-deficient cells have greatly diminished respiratory capacity and reduced cell growth. In addition, the mitochondrial population shows heterogeneous properties, including wide variations in membrane potential, mitochondrial DNA content and protein constituents. Based on these observations, we propose that mitochondria do not function well as autonomous organelles and that the dynamic properties of mitochondria are inherently important for organellar integrity. In normal cells, high rates of fusion and fission enable mitochondria to cooperate with each other through continual exchange of contents. Individual mitochondria can show stochastic fluctuations in essential components, but such fluctuations are short-lived, because mitochondrial fusion will result in mixing and normalization of components. In cells lacking mitochondrial fusion, such restoration of components cannot occur, and defective mitochondria accumulate. We discovered that, in the absence of fusion, many mitochondria lack mitochondrial DNA (mtDNA) nucleoids. This defect explains the respiratory chain and membrane potential aberrations found in fusion-deficient cells.
Although the machineries mediating mitochondrial fusion and fission are being elucidated, much more needs to be understood about how mitochondrial dynamics is regulated. Dramatic changes in mitochondrial dynamics occur with cellular stress, activity, apoptosis, and disease. We are using biochemical and genetic approaches to identify cellular processes that regulate the activity of the mitochondrial fusion and fission complexes.
Learn more about our other research areas:



